Extract Monographs
Asian Ginseng
Asian ginseng (Panax ginseng C.A. Meyer) has the reputation of being the most important herb used in Chinese medications, “the ultimate elixir of life, a symbol of strength and long life, the source of happiness, a tonic and an aphrodisiac”. According to Traditional Chinese Medicine, Panax ginseng can reinforce qi, improve circulation, increase blood supply, revitalizes, aid recovery from weakness after illness and strengthen the immune system [1].
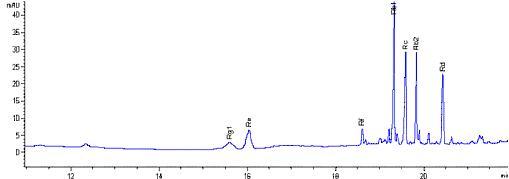
The active constituents of ginseng roots, known as ginsenosides (triterpenoid saponin glycosides), contribute primarily to the pharmacological effects. More than 30 ginsenosides have been identified in Panax species, but Rb1, Rb2, Rc, Rd, Re and Rg1 are the six major ginsenosides that represent over 90% of the total ginsenoside content in ginseng roots, with Rf used for identification of Asian ginseng.
Based on our research, the ratio of ginsenosides is different in the various plant parts, so the ginsenoside profile can effectively identify the different ginseng species and ginseng plant parts [3, 4]. To accommodate this, CPC has successfully developed a rapid and effective method to fully separate seven major ginsenosides in Asian Ginseng for both quantitative and qualitative analysis in less than 5 minutes with Zorbax Eclipse XDB-C18 column [3]. Therefore, by combining the reliable source of the raw herb and advanced processing method, the individual and total ginsenosides contents of our Canphy® Asian Gienseng P.E. have been strictly controlled in every batch. They meet USP standard with the ratio of Rb2 and Rb1 more than 0.4 and the total ginsenosides content is strictly controlled at 10%.
References
[1] State Commission of Chinese Pharmacopoeia of People’s Republic of China, Part Ⅰ, Chemical Industry Press, Beijing, 2005, PP. 205-206.
[2] Ma Yuan-Chun, Zhu J, Benkrima L, Luo M, Sun LH, Sain et al.. A comparative evaluation of ginsenosides in commercial ginseng products and tissue culture samples using HPLC. Journal of Herbs, Spices and Medicinal Plant (1995) 3, 41-50.
[3] Ma yuanchun, Luo mai, Lynn Malley, Monique Doucet. Distribution and proportion of major ginsenosides and quality control of ginseng products. Chinese Journal of Medicinal Chemistry (1996) 1, 11-20.
[4] Jie Ma, Yuan-Chun Ma, Daniel Wang, Fei Fei Hou, et al. Simultaneous Quantification of Panax and Epimedium Species Using Rapid Resolution Liquid chromatography (RRLC). Natural Product Communications (2011) 5, 581-586.